Just a few weeks ago, the National Council for Embryology and Genetic Research (NCEGR) granted a research team from the Orion Institute for Genomic Innovation in Northbridge a license to alter the genetic make-up of human embryos using a powerful gene editing tool known as CRISPR/Cas9. Although these embryos will never develop beyond the blastocyst stage and are not intended for implantation or birth, the approval has sparked widespread public and scientific debate. Lab Times investigated why this project relies specifically on human embryos and what scientists hope to learn from such controversial research.
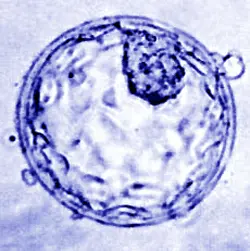
For more than a decade, Dr. Mira Kaelen, head of developmental genetics at the Orion Institute, has been exploring the earliest moments of human embryo development. One of the first major milestones in this process is the formation of the blastocyst, a hollow structure made up of around 200 cells. Within this structure lie about 20 epiblast cells the stem cells that will eventually give rise to the entire fetus. “Our research focuses on identifying the genetic pathways that instruct certain cells to become epiblast cells instead of placental or yolk sac cells,” said Dr. Kaelen at a recent press briefing.
Initially, Kaelen’s team relied on mouse embryo models for this work. However, they soon discovered that many key developmental genes behave very differently in human vs. mouse embryos. This discrepancy led them to ask a critical question: could these molecular differences explain why human reproduction is far less efficient than that of mice? While nearly all fertilized mouse embryos implant successfully, only around 25% of human embryos do.
To find answers, the team is employing CRISPR/Cas9 genome editing to remove a gene known as Oct4, which is active specifically in the epiblast. By knocking out Oct4, they expect that the embryos will fail to develop these critical stem cell populations, making it impossible to derive pluripotent human embryonic stem cells. “One of the assays we’re using involves attempting to derive stem cell lines from the edited embryos,” explained Kaelen. “If no stem cells can be created, that tells us Oct4 is essential for proper epiblast development.”
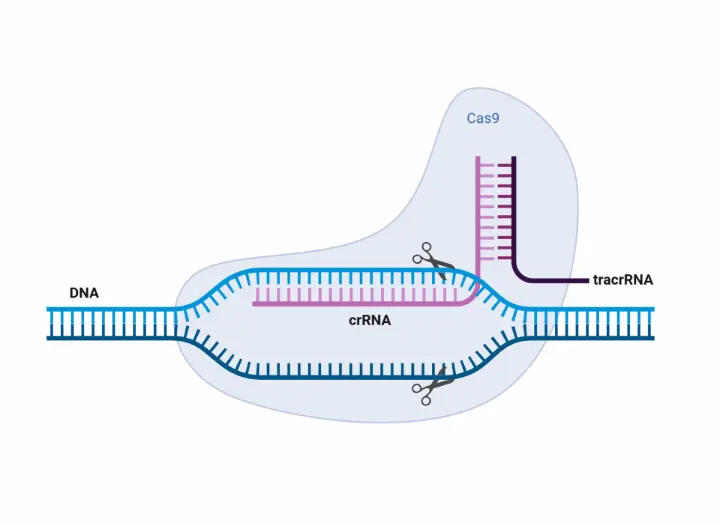
This is just the beginning. Once the Oct4 experiments are complete, the team plans to investigate two or three other genes that are uniquely expressed in early-stage human embryos and have not yet been functionally characterized. With CRISPR success rates exceeding 80% in previous mouse studies, they are confident in adapting the technology to human embryo models. Since Oct4 is highly conserved across species, the researchers believe they can target similar genomic regions for optimization.
What might this research lead to? Kaelen is cautious not to promise too much. “If we uncover the reasons human embryos fail to implant and find ways to make them more robust more like mouse embryo it could potentially improve fertility treatments, but that’s still speculative,” she said. “Right now, our goal is to better understand the fundamental genetic mechanisms of early development, which could benefit both stem cell biology and regenerative medicine.”
The Orion Institute team is ready to begin as soon as they receive enough donated embryos from their partnered IVF clinics across the Federation of Norwyn. To properly study each target gene, they estimate they’ll need between 20 and 30 zygotes per gene, allowing for adequate replication and analysis.
As CRISPR/Cas9 technology continues to evolve, this project could mark a turning point in human developmental biology, helping to bridge the gap between basic science and clinical breakthroughs.